
Ethylcellulose Aqueous Dispersion: Premium Film-Former for Coatings
Introduction to Ethylcellulose Aqueous Dispersion and Industry Trends
In the dynamic landscape of industrial coatings, pharmaceuticals, and specialized functional materials, the demand for advanced polymeric solutions capable of precise control over material properties is paramount. One such critical innovation is Ethylcellulose Aqueous Dispersion. This polymer derivative, widely recognized for its robust film-forming capabilities, water insolubility, and pH-independent release characteristics, offers unparalleled versatility in numerous B2B applications.
Current industry trends highlight a significant shift towards sustainable, solvent-free, and high-performance solutions. This movement is largely driven by stringent environmental regulations, growing consumer preference for safer products, and the continuous pursuit of enhanced product efficacy and shelf-life. Specifically, in pharmaceuticals, there's an escalating need for controlled drug release mechanisms, taste masking, and moisture barriers that improve bioavailability and patient compliance. In food science, the encapsulation of sensitive ingredients, shelf-life extension, and protective coatings for supplements are critical areas. For industrial coatings, the push for low VOC (Volatile Organic Compound) formulations, coupled with superior barrier properties and durability, has positioned Ethylcellulose Aqueous Dispersion as a preferred choice over traditional organic solvent-based systems. Its non-ionic nature and compatibility with a wide range of active pharmaceutical ingredients (APIs) and other excipients further solidify its role as an indispensable material in advanced formulation strategies.
Manufacturing Process Flow of Ethylcellulose Aqueous Dispersion
The production of Ethylcellulose Aqueous Dispersion involves a meticulously controlled multi-stage process designed to achieve consistent particle size, stability, and film-forming properties crucial for high-performance applications. The primary raw material is high-purity cellulose, typically sourced from wood pulp or cotton linters. The overall process is a testament to precision chemical engineering, ensuring the final product meets rigorous industry standards like ISO 9001 and relevant pharmacopoeial monographs (e.g., USP, EP, JP).
Process Flow Overview:
- Cellulose Ethoxylation: Raw cellulose is first treated with an alkalizing agent (e.g., NaOH) to create alkali cellulose. This activated cellulose then reacts with ethyl chloride in a controlled environment to produce ethylcellulose. This critical step determines the ethoxyl content, which directly impacts the polymer's solubility and film properties.
- Washing and Purification: The crude ethylcellulose undergoes extensive washing to remove residual salts, unreacted reagents, and undesirable by-products. This purification is vital for ensuring compliance with pharmaceutical and food-grade standards, minimizing impurities, and optimizing polymer performance.
- Dissolution and Emulsification: The purified ethylcellulose polymer, which is water-insoluble, is then dissolved in a suitable organic solvent. This solution is subsequently emulsified into water using high-shear mixing, often in the presence of a non-ionic emulsifying agent. The goal is to create a stable oil-in-water emulsion where the ethylcellulose is finely dispersed.
- Solvent Removal (Evaporation/Distillation): The organic solvent is then carefully removed from the emulsion through a controlled evaporation or distillation process. This leaves behind a stable aqueous dispersion of sub-micron ethylcellulose particles. This stage is crucial for achieving a low VOC profile and ensuring the safety of the final product. Advanced techniques such as spray drying can also be employed for creating powder forms.
- Filtration and Standardization: The resulting aqueous dispersion is filtered to remove any agglomerates or particulates, ensuring a uniform and consistent product. It then undergoes rigorous quality control testing for parameters like solids content, viscosity, pH, particle size distribution, and microbial limits. Batches are standardized to meet specific product specifications.
- Packaging: The finished product is packaged into sterile, appropriately sealed container111s, ready for distribution to target industries such as pharmaceuticals, food and beverage, specialty coatings, and agrochemicals.
This meticulous process ensures that the service life of products incorporating Ethylcellulose Aqueous Dispersion is maximized, offering superior stability, corrosion resistance in sensitive applications, and enhanced energy saving through efficient controlled release mechanisms. Its low permeability to water vapor and film integrity contribute significantly to the longevity and performance of final products.

Technical Specifications and Parameters
The performance of Ethylcellulose Aqueous Dispersion is defined by a precise set of technical parameters that are critical for its diverse applications. Understanding these specifications allows formulators and engineers to select the optimal grade for their specific requirements, ensuring product efficacy and manufacturing efficiency. Key parameters include ethoxyl content, solids content, viscosity, particle size distribution, and film-forming properties.
Typical Product Specifications
| Parameter | Unit | Value Range (Typical) | Significance in Application |
|---|---|---|---|
| Solids Content | % w/w | 28 - 32 | Dictates coating efficiency and dry film thickness per application. Higher content reduces drying time and passes. |
| Viscosity (Brookfield, 20°C) | mPa·s | 100 - 400 | Crucial for spray coating consistency, atomization, and film formation. Influences processability. |
| pH Value | 3.5 - 5.5 | Ensures dispersion stability and compatibility with pH-sensitive APIs or substrates. | |
| Particle Size (D50) | nm | 200 - 500 | Impacts film smoothness, transparency, and mechanical properties. Finer particles lead to superior films. |
| Glass Transition Temperature (Tg) | °C | 43 - 50 | Influences film flexibility and mechanical strength. Relevant for post-coating handling and storage stability. |
| Ethoxyl Content | % | 44 - 50 | Determines water resistance, flexibility, and overall barrier properties of the film. |
| Minimum Film-Forming Temperature (MFFT) | °C | < 20 | Indicates the lowest temperature at which a continuous film can be formed. Lower MFFT allows for room temperature processing. |
These parameters are rigorously tested using international standards (e.g., ASTM, ISO, Pharmacopoeial methods) to ensure batch-to-batch consistency and predictable performance. The optimal selection of Ethylcellulose Aqueous Dispersion grade depends directly on the specific application's requirements, such as desired release profile, film flexibility, and processing conditions.
Application Scenarios Across Key Industries
The unique physicochemical properties of Ethylcellulose Aqueous Dispersion make it an indispensable material across a spectrum of industries, particularly where controlled release, protection, and enhanced functionality are required. Its ability to form a strong, water-insoluble, yet flexible film, coupled with its non-toxic profile, positions it as a versatile solution for complex B2B challenges.
Pharmaceutical Industry:
- Controlled Release Coatings: For oral solid dosage forms (tablets, pellets, granules), it provides precise control over drug release kinetics, enabling sustained or delayed-release profiles. This optimizes therapeutic efficacy and reduces dosing frequency.
- Taste Masking: Effectively masks bitter or unpleasant tastes of APIs, significantly improving patient compliance, especially in pediatric and geriatric populations.
- Moisture Barriers and Protection: Protects moisture-sensitive APIs from degradation, extending shelf-life and maintaining product stability in varying environmental conditions.
- Barrier for Effervescent Tablets: Prevents premature reaction with moisture, preserving the integrity and performance of effervescent formulations.
Food and Dietary Supplements Industry:
- Encapsulation of Flavors and Nutrients: Protects volatile flavors, vitamins, and sensitive functional ingredients from oxidation, moisture, and degradation, thereby extending their shelf-life and ensuring potency.
- Food Coatings: Applied to confectionery, nuts, and dried fruits to provide moisture barrier, prevent sticking, and enhance gloss and appearance.
- Controlled Release of Active Ingredients: In functional foods or pet food, it can regulate the release of active components, similar to pharmaceutical applications.
Specialty Coatings and Agrochemicals:
- Barrier Coatings: Utilized in various industrial coatings for its excellent moisture resistance and film strength, offering protection against environmental degradation.
- Seed Coatings: In agriculture, it provides a protective layer for seeds, allowing for the controlled release of pesticides, fungicides, or growth promoters, thus optimizing crop yield and reducing environmental impact.
- Micronutrient Encapsulation: For foliar sprays or soil amendments, it can encapsulate micronutrients or beneficial microorganisms, ensuring their sustained availability and efficacy.

Technical Advantages and Performance Benefits
The selection of Ethylcellulose Aqueous Dispersion over alternative film-forming polymers is driven by a compelling suite of technical advantages that directly translate into superior product performance and process efficiency for B2B clients. These benefits are rooted in its unique polymeric structure and the sophisticated manufacturing process that yields a high-quality, stable dispersion.
- Excellent Water Barrier Properties: Forms highly effective films that are virtually impermeable to water and water vapor. This is critical for protecting hygroscopic active ingredients, extending product shelf-life, and preventing degradation from moisture ingress. Data shows it can reduce moisture uptake by up to 70% compared to uncoated materials in high humidity environments.
- pH-Independent Release: Unlike many anionic polymers that are pH-sensitive, ethylcellulose films maintain their integrity and release profile regardless of gastrointestinal pH. This provides consistent drug release across the entire digestive tract, enhancing predictability and efficacy.
- Mechanical Strength and Flexibility: Films formed from Ethylcellulose Aqueous Dispersion exhibit high tensile strength and flexibility. This prevents cracking, chipping, or peeling during handling, packaging, and storage, ensuring the integrity of coated products. Its elongation at break can exceed 10-15%, demonstrating superior robustness.
- Non-Tacky Film Formation: Dries to a smooth, non-tacky surface, which is crucial for preventing coated products from sticking together during processing, packaging, and storage. This improves manufacturing throughput and reduces product waste.
- Low Viscosity at High Solids Content: Allows for efficient application (e.g., spray coating) at higher solids concentrations, reducing the number of coating passes required and decreasing drying times. This leads to significant energy savings and increased productivity.
- Excellent Pigment Compatibility: Its non-ionic nature ensures excellent compatibility with a wide range of pigments and other excipients, enabling the production of vibrant, aesthetically pleasing, and stable colored coatings without stability issues.
- Environmentally Friendly (Aqueous System): Being an aqueous dispersion, it eliminates the need for organic solvents, reducing VOC emissions and aligning with green chemistry principles. This provides a safer working environment and simplifies regulatory compliance.
- Biocompatibility and Safety: As a cellulose derivative, it is generally recognized as safe (GRAS) by regulatory bodies like the FDA, making it suitable for pharmaceutical and food-contact applications.
These technical merits underscore why Ethylcellulose Aqueous Dispersion remains a preferred choice for formulators seeking to achieve high-performance, cost-effective, and regulatory-compliant solutions.
Vendor Comparison and Customized Solutions
Selecting the right supplier for Ethylcellulose Aqueous Dispersion is as critical as understanding its technical merits. The market offers various options, but significant differences exist in product quality, technical support, and customization capabilities. A robust vendor partnership ensures not only consistent supply but also access to expertise that can optimize application development and troubleshooting.
Key Differentiators in Vendor Selection:
| Feature | Standard Offerings | Premium/Specialized Offerings (Our Advantage) |
|---|---|---|
| Product Quality & Consistency | Standard grades, basic QC. Potential batch variability. | Pharmacopoeial (USP/EP/JP) compliant, ISO 9001 certified. Ultra-low VOC. Consistent particle size distribution (PSD) verified by DLS. |
| Technical Support | Basic product data sheets, limited application guidance. | Dedicated R&D team for formulation optimization, on-site technical assistance, comprehensive application guides, and troubleshooting. |
| Customization Capabilities | Limited options, typically only standard concentrations. | Tailored solids content, viscosity adjustments, specialized excipient blends, modified MFFT for specific processing conditions, and custom packaging. |
| Regulatory Compliance | May meet basic industry standards. | Full documentation support (CoA, MSDS, TSE/BSE statements), GMP manufacturing, FDA Drug Master File (DMF) support where applicable. |
| Supply Chain Reliability | Standard lead times, less flexibility for urgent orders. | Robust global logistics network, established inventory management, flexible production scheduling, emergency order fulfillment capabilities. |
Our commitment extends beyond providing a commodity. We partner with clients to develop truly customized solutions using Ethylcellulose Aqueous Dispersion that address their unique formulation and manufacturing challenges. This collaborative approach involves understanding specific product requirements, desired performance characteristics, and processing constraints.
Customization options include fine-tuning the solids content for different coating processes, adjusting viscosity for specific spray equipment, incorporating specialized plasticizers for enhanced film flexibility, or tailoring the particle size distribution to achieve specific release profiles or surface finishes. Our R&D team, with decades of experience in polymer science, works closely with client engineers and formulators to deliver bespoke solutions that optimize performance, reduce development cycles, and ensure regulatory adherence.
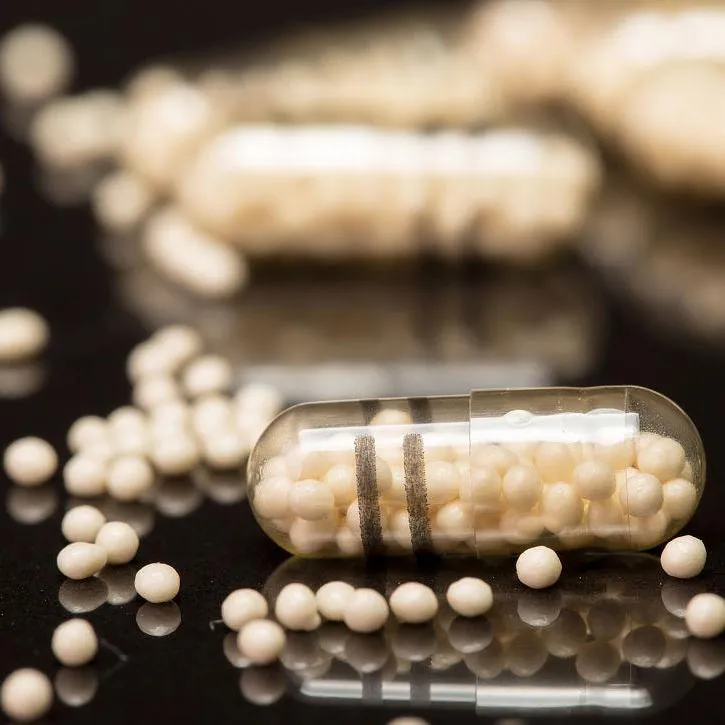
Application Case Studies
Real-world applications demonstrate the tangible benefits and versatility of Ethylcellulose Aqueous Dispersion in solving complex industrial challenges. These case studies highlight our commitment to delivering high-performance, tailored solutions.
Case Study 1: Enhanced Stability for Hygroscopic Pharmaceutical Tablets
Client Challenge: A pharmaceutical manufacturer faced significant stability issues with a new moisture-sensitive API. Tablets were exhibiting degradation and reduced potency due to ambient humidity, leading to shortened shelf-life and high rejection rates. Traditional film coatings provided insufficient moisture barrier.
Solution: Our team collaborated with the client to develop a bespoke coating formulation utilizing a high-solids Ethylcellulose Aqueous Dispersion. We optimized the application parameters (e.g., spray rate, inlet air temperature) to achieve a uniform, defect-free film with superior barrier properties.
Results & Benefits: The coated tablets demonstrated a 65% reduction in moisture uptake over a 6-month accelerated stability study, extending the product's shelf-life by 12 months. This led to a 30% reduction in manufacturing waste and enabled market entry in high-humidity regions, significantly increasing market reach.
Case Study 2: Controlled Release for Agrochemical Granules
Client Challenge: An agrochemical company needed a coating for their pesticide granules that would ensure a sustained release of the active ingredient over 4-6 weeks, reducing the frequency of application and minimizing environmental leaching. Current coatings failed to provide a consistent, long-term release profile.
Solution: We provided a customized Ethylcellulose Aqueous Dispersion formulation with a specific ethoxyl content and particle size optimized for slow erosion and diffusion-controlled release. Our technical support team guided the client through coating pan equipment calibration and process validation.
Results & Benefits: The coated granules achieved the desired 5-week sustained release profile, confirmed by dissolution testing in simulated soil conditions. This resulted in a 25% reduction in pesticide usage, lower labor costs for farmers, and significant environmental benefits through reduced active ingredient runoff. Customer feedback highlighted improved crop yields and reduced application frequency.
Case Study 3: Taste Masking for Pediatric Drug Formulation
Client Challenge: A pediatric pharmaceutical company developed a liquid formulation of a vital drug with an extremely bitter taste, leading to poor patient compliance. They sought an effective taste-masking solution compatible with their liquid suspension format.
Solution: We recommended a specialized fine-particle Ethylcellulose Aqueous Dispersion to microencapsulate the active ingredient before incorporation into the liquid suspension. Our experts assisted in optimizing the coacervation process to ensure complete coating of the API particles, effectively sealing off the bitter taste receptors.
Results & Benefits: Sensory evaluation and patient feedback confirmed a significant improvement in taste palatability, with the majority of children accepting the medication without objection. This led to a projected 40% increase in patient adherence and successful market launch of the new pediatric drug, enhancing the company's reputation and market share.

Trustworthiness Module: FAQ, Lead Time, Warranty & Support
Our commitment to our B2B partners extends beyond product delivery to encompass comprehensive support, transparency, and reliability, fostering long-term trust and successful collaboration.
Frequently Asked Questions (FAQ)
-
Q: What is the recommended storage condition for Ethylcellulose Aqueous Dispersion?
A: It should be stored in tightly sealed container111s at temperatures between 5°C to 30°C, protected from freezing and direct sunlight. Proper storage ensures its stability and prevents microbial contamination. -
Q: Is your Ethylcellulose Aqueous Dispersion compliant with major pharmacopoeias?
A: Yes, our products are manufactured to meet or exceed the requirements of leading pharmacopoeias, including USP (United States Pharmacopeia) and EP (European Pharmacopoeia), confirmed by comprehensive Certificates of Analysis (CoA). -
Q: Can I dilute the dispersion, and if so, how?
A: Yes, the dispersion can be diluted with deionized water to achieve desired solids content. It is recommended to add water slowly with continuous, gentle agitation to maintain dispersion stability and homogeneity. -
Q: What is the typical lead time for orders?
A: Standard orders typically have a lead time of 2-4 weeks, depending on volume and specific product grade. Expedited options are available upon request for urgent requirements. We maintain strategic inventory levels to ensure timely fulfillment.
Lead Time and Fulfillment
We pride ourselves on efficient logistics and a robust supply chain. Our standard lead time for Ethylcellulose Aqueous Dispersion is 10-14 business days for typical order volumes, with expedited options available for urgent requirements. For customized formulations or large-scale project-based orders, lead times will be mutually agreed upon based on the complexity and scale of production. We utilize a global distribution network to ensure reliable and timely delivery worldwide.
Warranty Commitments
We stand behind the quality of our Ethylcellulose Aqueous Dispersion products. All products are warranted to meet the published specifications and quality standards for a period of 12 months from the date of manufacture when stored and handled according to our recommendations. In the unlikely event of a product not meeting these specifications, we commit to prompt investigation, replacement, or credit, ensuring client satisfaction and minimizing disruption.
Customer Support and Technical Assistance
Our dedicated technical support team comprises experienced polymer chemists and application specialists ready to assist with formulation development, process optimization, and troubleshooting. We offer:
- Expert Consultation: Guidance on product selection and application.
- Laboratory Services: Access to our state-of-the-art labs for compatibility testing and performance evaluation.
- On-Site Support: For complex projects or process scale-up.
- Comprehensive Documentation: Full regulatory dossiers, Certificates of Analysis (CoA), and Material Safety Data Sheets (MSDS).
Contact our support team at [info@tangzhihpmc.com] or visit our website for further assistance.
Conclusion
Ethylcellulose Aqueous Dispersion stands as a cornerstone material for advanced B2B applications, offering unmatched performance in controlled release, moisture barrier, and film-forming capabilities. Its versatility, coupled with its environmentally friendly aqueous nature, positions it as an essential component in pharmaceutical, food, and specialized coating industries. Our dedication to superior product quality, robust technical support, and tailored solutions ensures that clients can achieve optimal product performance and competitive advantage.
Through continuous innovation and stringent quality control, we remain committed to empowering our partners with the high-quality materials and expertise necessary to excel in their respective markets.
References
- Rowe, R. C., Sheskey, P. J., & Quinn, M. E. (2009). Handbook of Pharmaceutical Excipients. Pharmaceutical Press.
- European Pharmacopoeia (Ph. Eur.) Monographs. Council of Europe.
- United States Pharmacopeia (USP) and National Formulary (NF). U.S. Pharmacopeial Convention.
- Siepmann, J., & Siepmann, F. (2008). Mathematical modeling of drug dissolution. International Journal of Pharmaceutics, 364(2), 272-283.
- Liu, S., & Li, R. (2008). Applications of Ethylcellulose in pharmaceutical dosage forms. Journal of Drug Delivery Science and Technology, 18(4), 281-289.
-
Cellulose Fiber Suppliers | Sustainable Natural Fibers for Industry and InnovationNewsNov.20,2025
-
Reliable Hydroxypropyl Methylcellulose Suppliers for Pharmaceutical & Industrial Use | TangzhiNewsNov.20,2025
-
Leading Cellulose Insulation Supplier for Sustainable Building MaterialsNewsNov.19,2025
-
Trusted Hydroxypropyl Cellulose Suppliers for Quality & SustainabilityNewsNov.18,2025
-
Top Cellulose Acetate Suppliers for Sustainable Industrial SolutionsNewsNov.18,2025
-
Reliable HPMC Suppliers for Sustainable Industrial Solutions | TangzhiNewsNov.18,2025





















