Hydroxypropyl Methylcellulose Phthalate
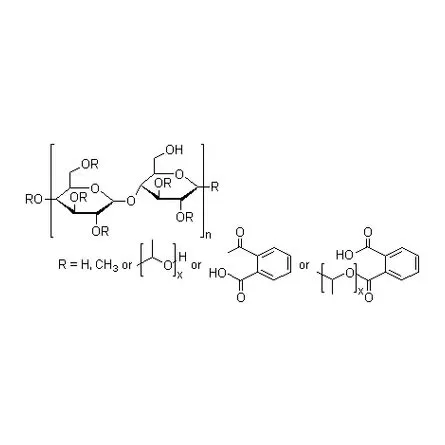
HPMCP is an enteric film coating material with excellent performance. It is mainly used as enteric coating material and sustained-release skeleton material for tablets and granules, microcapsule matrix, and other equipment. Its release rate is related to pH value.
HPMCP is a white or off-white powder that is tasteless and odorless.
It has good solubility in organic solvents but is insoluble in water, which makes it particularly suitable for formulations that require controlled dissolution or release properties.

|
Item Specification |
HP55 |
HP55S |
HP50 |
|
Viscosity%, 20±0.1°C (mpa.s) |
32-48 |
136-204 |
44-46 |
|
moisture(wt%) |
≤5.0 |
||
|
Ignition residue (wt%) |
≤0.2 |
||
|
Oxide (wt%) |
≤0.07 |
||
|
Arsenic (ppm) |
≤2 |
||
|
Heavy metals (ppm) |
≤10 |
||
|
Free phthalic acid (wt%) |
≤1.0 |
||
|
Phthaloyl (wt%) |
21.0-35.0 |
21.0-27.0 |
|
|
Methoxy (wt%) |
18.0-22.0 |
20.0-24.0 |
|
|
Hydroxypropoxy (wt%) |
5.0-9.0 |
6.0-10.0 |
|
In compliance with the 2020 edition of the Chinese Pharmacopoeia (Part IV);Registration Number: F20180000536
Note: HP55S has higher molecular weight, stronger film strength and stronger resistance to human gastric juice.
It has plasticity and good film-forming properties. No or less plasticizer is needed during coating, which can improve the appearance and strength of tablets. It can also be used with a small amount of shellac (80:20) to enhance resistance to gastric juice.
Its dissolution pH is lower than that of acrylic resin (PH5.0-5.5), the drug dissolves quickly, and the bioavailability is high.
HPMCP has good storage stability and will not deteriorate for 3-4 years under storage conditions. Its effect on drug release and absorption is smaller than that of acrylic resin.
HPMCP is better than cellulose acetate phthalate as an enteric film material, and because its chemical structure does not contain acetate groups, it will not release acetic acid during storage, causing drug deterioration like CAP.
The ammonium salt polymer of HPMCP is one of the main components of enteric capsules. The gelatin prepared with HPMCP or gelatin can form a tough and transparent film that dissolves or disintegrates in the intestine.

1.Appearance: This product is white or off-white powder or granules, odorless and tasteless.
2.True specific gravity: 1.65-1.82
3.Apparent density: 0.2-0.4g/cm
4.Solubility in organic solvents: This product is soluble in solvents such as acetone, acetone/water(95:5), acetone/ethanol (1:1), methanol/dichloromethane (1:1),acetone/methanol (1:1) dichloromethane/ethanol (1:1), ethanol/water (8:2), etc., but it is not soluble in water, anhydrous ethanol, or acidic buffer, and is very slightly soluble in toluene.
5.HP55S has a higher molecular weight than HP55, and has stronger film strength and stronger resistance to artificial gastric juice.
6.HP55 is soluble in buffer media with a pH value of more than 5.5, and HP50 is soluble in buffer media with a pH value of more than 5.0.
7.Water absorption: absorbs 25% water at room temperature.
8.Softening point: 200-210°C.
The concentration of the coating solution
|
Tablet |
Granules |
|
|
HP55 |
6%-10% |
5%-7% |
|
HP55S |
5%-8% |
4%-6% |
Organic solvents used in HP
|
Acetone / water |
9 : 1 |
|
Dichloromethane / ethanol |
1 : 1 |
|
Acetone / ethanol |
1 : 1 |
|
Ethanol / water |
8 : 2 (HP55) ; 8.5 : 1.5 (HP55S) ;7 : 8 (HP50)Solution temperature above 25℃ |
|
Dichloromethane /ethanol /water |
5 : 4 : 1 |
|
Acetone / water |
9 : 1 |
Dissolution method
Add a certain amount of solvent into the container, and slowly add HPMCP while stirring until viscosity is completely formed.
The dosage of HPMCP is generally 6-10% of the tablet core.
HPMCP is an enteric film coating material with excellent performance. It is mainly used as enteric coating material and sustained-release skeleton material for tablets and granules, enteric capsule adhesive, microcapsule matrix, implant, gradient drug release system, taste masking agent, microsphere, oral membrane, solid dispersion and other equipment. Its release rate is related to pH value.
Packaged in a cardboard barrel lined with a polyethylene bag, net weight: 10.0kg/barrel.
Store in a cool, dry and ventilated place, and be waterproof and moisture-proof, avoid high temperature and direct sunlight.











