Hydroxypropyl Methylcellulose Acetate Succinate
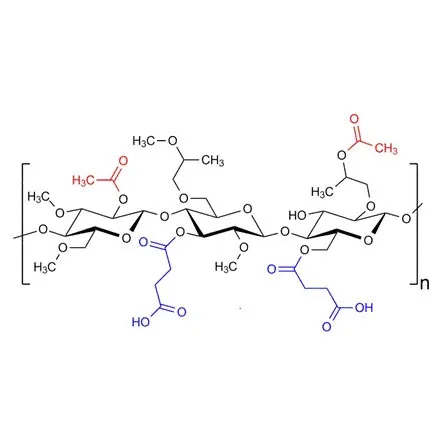
Hydroxypropyl Methylcellulose Acetate Succinate (HPMCAS) is a semi-synthetic polymer derived from cellulose, widely used in pharmaceutical formulations due to its unique properties.
It is usually a white to off-white powder. HPMCAS can increase the solubility and bioavailability of poorly soluble drugs, thereby improving drug absorption efficiency. It can be used to prepare sustained-release and controlled-release drug preparations to help adjust the drug release rate to achieve the best therapeutic effect. HPMCAS is an extremely important and multifunctional excipient in the pharmaceutical field.

|
Item |
Index |
|
Viscosity mpa.s |
20-5000 |
|
Loss on drying (%) |
≤5.0 |
|
Residue on ignition (%) |
≤0.2 |
|
Arsenic (ppm) |
≤2.0 |
|
Heavy metals (ppm) |
≤10.0 |
|
Acetic acid and succinic acid (%) |
≤1.0 |
|
Acetyl (%) |
2.0-16.0 |
|
Succinyl (%) |
4.0-28.0 |
|
Methoxy (%) |
12.0-28.0 |
|
2-hydroxypropoxy (%) |
4.0-23.0 |
Conforms to the standards of the Chinese Pharmacopoeia 2020 edition (Volume 4)
Model and specifications
|
Model |
Acetyl content |
Succinyl content |
Average particle size |
Marking viscosity |
|
|
Micro powder type |
AS-LF |
8 |
15 |
5 μm |
3 mm²/s |
|
AS-MF |
9 |
11 |
|||
|
AS-HF |
12 |
6 |
|||
|
Granular type |
AS-LG |
8 |
15 |
1 ㎜ |
|
|
AS-MG |
9 |
11 |
|||
|
AS-HG |
12 |
6 |
|||
True specific gravity: 1.27-1.30
Apparent density: (g/ml, 20°C)
Type F: loose 0.2-0.3, tapped 0.3-0.5
Type G: loose 0.2-0.5, tapped 0.3-0.6

Film-forming property: after the solvent evaporates, a smooth and dense film is left. The film has good transparency, is smooth and dense, is stable in gastric juice for 3.5 hours, does not bubble, does not turn white, and does not dissolve. It completely dissolves in intestinal juice within 30 minutes.
Solubility: easily soluble in methanol, acetone, acetone/ethanol, acetone/methanol, methanol/dichloromethane, ethanol/dichloromethane (1:1wt) mixture, easily soluble in aqueous solution with a pH higher than 5.0, insoluble in water and acidic solution, insoluble in xylene and n-hexane.
AS-H 135
HPMCP 145
Glass transition temperature (℃):
|
AS-L |
120 |
|
AS-M |
130 |
|
AS-H |
135 |
|
HPMCP |
145 |
Membrane strength
|
AS - L |
AS - M |
AS - H |
|
|
Tensile strength Mpa |
52 |
51 |
55 |
|
Elongation % |
8.4 |
7.2 |
4.3 |
Water vapor permeability: HPMCAS has a low water vapor permeability, which means it effectively blocks the penetration of moisture. This property is particularly important for protecting drugs from humidity, especially in high humidity environments, to prevent drugs from absorbing moisture, agglomerating or degrading.
Hygroscopicity(75%RH):
|
AS-LF |
7-8% |
|
AS-MF |
6-7% |
|
AS-HF |
5-6% |
Thermal degradation temperature 200°C

Wet test chamber
*Constant temperature and humidity
HPMCAS is an enteric material that can be used as an enteric coating material, microcapsule membrane material, and sustained-release material, such as preparing sustained-release granules, tablets, capsules, and even microcapsules. When making sustained-release agents, its release rate is related to pH.
-
 01Enteric coating
01Enteric coatingHPMCAS is almost insoluble in the acid environment of the stomach, but can dissolve rapidly in a near neutral to weakly alkaline environment (such as the small intestine environment).
-
 02Sustained-release control agent
02Sustained-release control agentHPMCAS is used to prepare sustained-release and controlled-release drug preparations to help regulate the release rate of drugs, thereby optimizing therapeutic effects and reducing dosing frequency.
-
 03Solid dispersion carrier
03Solid dispersion carrierHPMCAS can increase the solubility and bioavailability of certain poorly soluble drugs and improve the absorption efficiency of drugs by forming solid dispersions.
-

 04Food packaging materials
04Food packaging materialsDue to its low water vapor permeability, HPMCAS can be used to make food packaging materials with high barrier properties, protecting food from moisture and extending shelf life.
Special Note
Environmental protection: As a polymer based on natural cellulose modification, HPMCAS is biodegradable, which makes it also have certain advantages in environmental protection.
Advantages
Compared with other enteric materials, HPMCAS has more stable storage conditions, dissolves faster in the intestine, and is less likely to react with coating materials. For example: CAP (cellulose acetate phthalate) is easily affected by high temperature and high humidity conditions. It will slowly hydrolyze after long storage, resulting in increased acidity and viscosity; in addition, CAP dissolves slowly in the intestine.
The production process of hydroxypropyl methylcellulose acetate succinate (HPMCAS) involves chemical modification of natural cellulose to introduce specific functional groups. The basic steps are as follows:
1.Raw material preparation:
Select high-quality cellulose raw materials, usually natural sources such as wood pulp or cotton linter.
2.Alkalinization treatment:
React cellulose with a strong base (such as sodium hydroxide) to produce alkali cellulose. This process is called alkalization or swelling, and the purpose is to open the hydrogen bond network between cellulose chains to make subsequent reactions easier.
3.Etherification reaction:
On the basis of alkali cellulose, methyl chloride and propylene oxide are added as etherifying agents and reacted under certain conditions. This step aims to introduce methoxy (-OCH₃) and hydroxypropoxy (-OCH₂CHOHCH₃) into the cellulose molecular chain to form preliminary hydroxypropyl methylcellulose (HPMC).
4.Esterification reaction:
Next, the HPMC obtained above is further treated by adding acetic anhydride and succinic anhydride to introduce acetyl (-COCH₃) and succinyl (-COCH₂CH₂COOH) respectively, thereby producing HPMCAS.
5.Neutralization and washing:
After the reaction is completed, the product needs to be neutralized to remove residual acid or other reaction by-products, and impurities are removed by multiple water washings to ensure the purity of the final product.
6.Drying and crushing:
The wet product after washing needs to be dried, usually by spray drying or drum drying. The dried product is crushed according to demand to achieve an appropriate particle size distribution.
7.Quality control:
The final product will undergo a series of quality tests, including determination of key indicators such as degree of substitution, solubility, viscosity, etc., to ensure that it meets the established standards.

Packaging with cardboard, lined with double-layer polyethylene plastic bags, 25kg/barrel, stored in a cool, dry and ventilated environment, and waterproof and moisture-proof, avoid high temperature and direct sunlight.










